A low-dose OC with
an innovative 4-phasic
dosing regimen2
For pregnancy prevention and the treatment of heavy menstrual bleeding (HMB) in women without organic pathology who choose to use an oral contraceptive (OC) as their method of contraception1
Natazia® is the only available low-dose OC with estradiol valerate (E2V) as part of a 28-day regimen1,3
Breaking down the
4 phases of Natazia
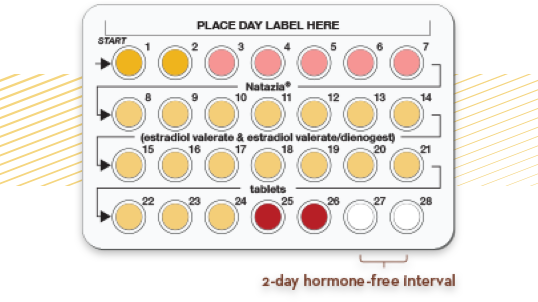

Phase 1 (Days 1-2) Encourages endometrial buildup with an initial 3-mg dose of E2V2

Phase 2 (Days 3-7) Slows endometrial growth by introducing a low dose of dienogest (DNG) (2 mg), while reducing E2V (2 mg)2

Phase 3 (Days 8-24) Stabilizes the endometrium by raising the DNG level (3 mg)2,4

Phase 4 (Days 25-26 and Days 27-28) Promotes a controlled menses by ending with a short interval of further-reduced E2V (1 mg) only, followed by a 2-day washout2,5,6


